How Will You Know if You Get a Racemic Mixture
v.8: Racemic Mixtures and the Resolution of Enantiomers
- Page ID
- 31427
Afterward completing this department, yous should exist able to
- describe a common process for separating a mixture of enantiomers.
- explain why racemic mixtures practice not rotate plane-polarized light.
Make sure that you tin define, and apply in context, the key terms beneath.
- racemic mixture (or racemate)
- resolve
A racemic mixture is a l:l mixture of two enantiomers. Because they are mirror images, each enantiomer rotates plane-polarized lite in an equal merely reverse direction and is optically inactive. If the enantiomers are separated, the mixture is said to have been resolved. A common experiment in the laboratory component of introductory organic chemical science involves the resolution of a racemic mixture.
The dramatic biochemical consequences of chirality are illustrated by the utilize, in the 1950s, of the drug Thalidomide, a sedative given to significant women to relieve morning sickness. It was later realized that while the (+)‑grade of the molecule, was a safe and effective sedative, the (−)‑form was an agile teratogen. The drug caused numerous birth abnormalities when taken in the early stages of pregnancy because information technology independent a mixture of the two forms.
Every bit noted earlier, chiral compounds synthesized from achiral starting materials and reagents are generally racemic (i.e. a l:50 mixture of enantiomers). Separation of racemates into their component enantiomers is a process chosen resolution. Since enantiomers take identical physical properties, such as solubility and melting indicate, resolution is extremely difficult. Diastereomers, on the other hand, have different physical backdrop, and this fact is used to accomplish resolution of racemates. Reaction of a racemate with an enantiomerically pure chiral reagent gives a mixture of diastereomers, which can be separated. For example, if a racemic mixture of a chiral booze is reacted with a enantiomerically pure carboxylic acrid, the effect is a mixture of diastereomers: in this instance, because the pure (R) entantiomer of the acid was used, the product is a mixture of (R-R) and (R-S) diastereomeric esters, which can, in theory, be separated by their different physical backdrop. Subsequent hydrolysis of each separated ester will yield the 'resolved' (enantiomerically pure) alcohols. The used in this technique are known as 'Moscher's esters', later on Harry Rock Moscher, a chemist who pioneered the method at Stanford University.
As noted before, chiral compounds synthesized from achiral starting materials and reagents are more often than not racemic (i.east. a 50:50 mixture of enantiomers). Separation of racemates into their component enantiomers is a process called resolution. Since enantiomers have identical concrete properties, such as solubility and melting point, resolution is extremely difficult. Diastereomers, on the other hand, have different physical backdrop, and this fact is used to attain resolution of racemates. Reaction of a racemate with an enantiomerically pure chiral reagent gives a mixture of diastereomers, which tin exist separated. Reversing the outset reaction then leads to the separated enantiomers plus the recovered reagent.
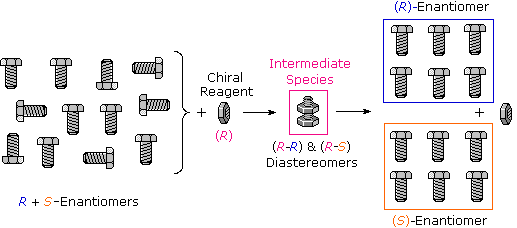
Many kinds of chemical and physical reactions, including salt germination, may exist used to accomplish the diastereomeric intermediates needed for separation. Figure 5.8.1 illustrates this general principle by showing how a nut having a right-handed thread (R) could serve every bit a "reagent" to discriminate and separate a mixture of right- and left-handed bolts of identical size and weight. Only the 2 right-handed partners can interact to give a fully-threaded intermediate, so separation is fairly simple. The resolving moiety, i.e. the nut, is then removed, leaving the bolts separated into their right and left-handed forms. Chemic reactions of enantiomers are normally not so dramatically different, but a practical stardom is nevertheless possible.
Because the physical properties of enantiomers are identical, they seldom can exist separated by simple physical methods, such as fractional crystallization or distillation. It is simply under the influence of another chiral substance that enantiomers behave differently, and almost all methods of resolution of enantiomers are based upon this fact. Nosotros include here a discussion of the principal methods of resolution.
Chiral Amines as Resolving Agents and Resolution of Racemic Acids
The nigh unremarkably used procedure for separating enantiomers is to convert them to a mixture of diastereomers that will have different concrete backdrop: melting betoken, boiling point, solubility, then on (Department 5-v). For example, if y'all take a racemic or R, S mixture of enantiomers of a carboxylic acrid and catechumen this to a salt with a chiral amine base having the R configuration, the salt will exist a mixture of two diastereomers, (R acid . R base) and (S acid . R base). These diastereomeric salts are not identical and they are not mirror images. Therefore they will differ to some caste in their concrete backdrop, and a separation by physical methods, such as crystallization, may be possible. If the diastereomeric salts can be completely separated, the carboxylic acid regenerated from each salt will be either exclusively the R or the S enantiomer.
Resolution of chiral acids through the formation of diastereomeric salts requires adequate supplies of suitable chiral bases. Brucine, strychnine, and quinine oftentimes are used for this purpose because they are readily available, naturally occurring chiral bases. Simpler amines of constructed origin, such as 2-amino- 1 -butanol, amphetamine, and 1 -phenylethanamine, also can be used, but offset they must be resolved themselves.
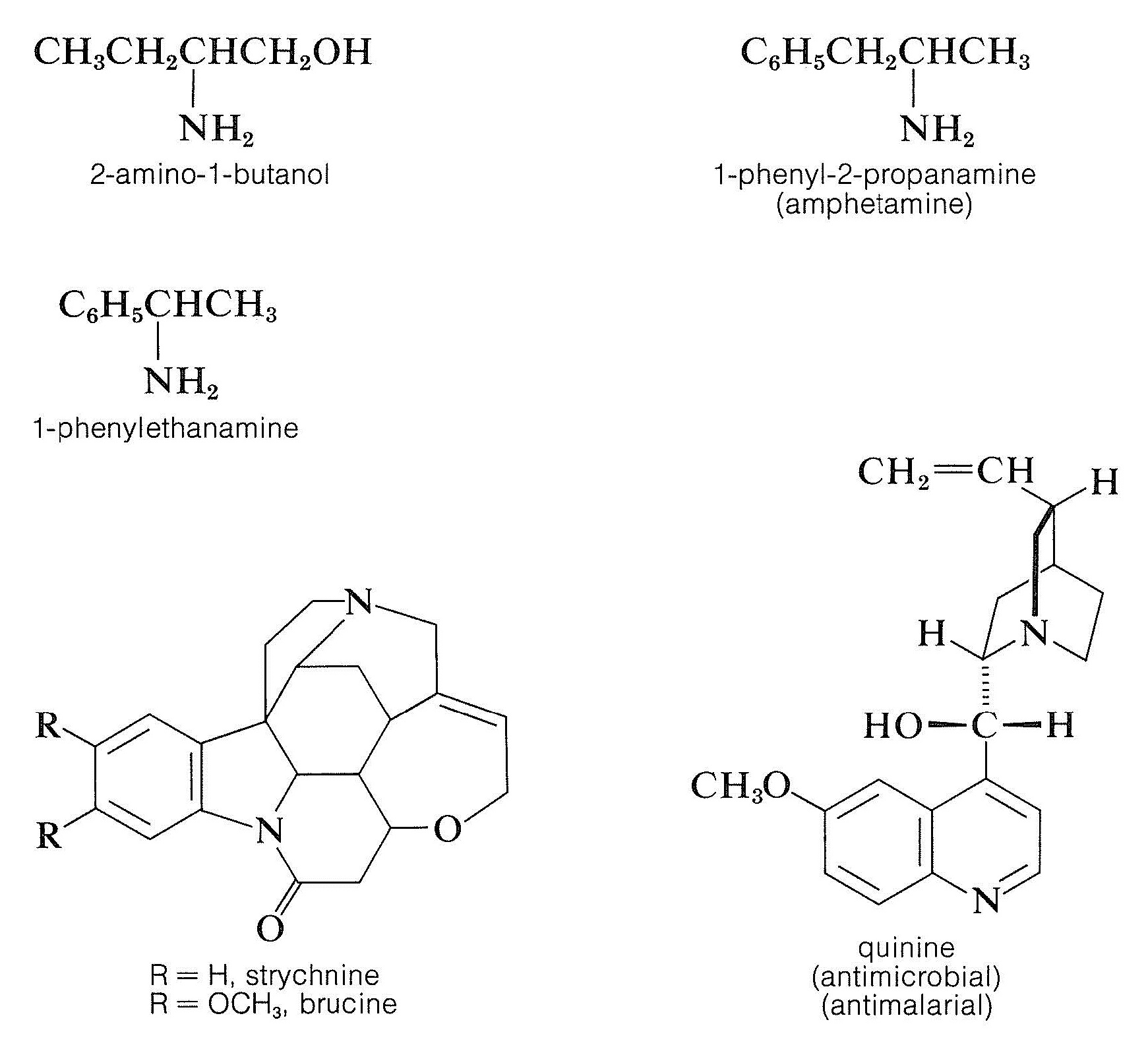
Show how (S)-1-phenylethylamine can be used to resolve a racemic mixture of lactic acid. Please depict all the structures involved.
- Answer
-
Resolution of Racemic Bases
Chiral acids, such as (+)-tartaric acrid, (-)-malic acid, (-)-mandelic acrid, and (+)-camphor- 10-sulfonic acid, are used for the resolution of a racemic base.
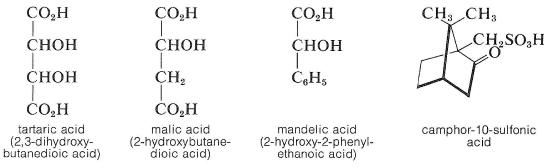
The principle is the same as for the resolution of a racemic acid with a chiral base of operations, and the pick of acid will depend both on the ease of separation of the diastereomeric salts and, of course, on the availability of the acrid for the calibration of the resolution involved. Resolution methods of this kind tin can be tedious, because numerous recrystallizations in different solvents may be necessary to progressively enrich the crystals in the less-soluble diastereomer. To decide when the resolution is consummate, the mixture of diastereomers is recrystallized until there is no further modify in the measured optical rotation of the crystals. At this stage it is hoped that the crystalline salt is a pure diastereomer from which i pure enantiomer tin can be recovered. The optical rotation of this enantiomer volition be a maximum value if it is "optically" pure considering any amount of the other enantiomer could only reduce the magnitude of the measured rotation \(\blastoff\).
Resolution of Racemic Alcohols
To resolve a racemic alcohol, a chiral acid can exist used to catechumen the alcohol to a mixture of diastereomeric esters. This is non as generally useful as might exist thought considering esters tend to be liquids unless they are very high-molecularweight compounds. If the diastereomeric esters are not crystalline, they must exist separated past some other method than partial crystallization (for instance, past chromatography methods, Department 9-2). Ii chiral acids that are useful resolving agents for alcohols are:
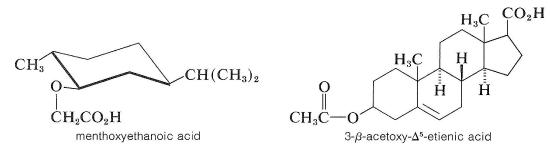
The nearly common method of resolving an alcohol is to convert it to a half-ester of a dicarboxylic acrid, such as butanedioic (succinic) or 1,2-benzenedicarboxylic (phthalic) acid, with the corresponding anhydride. The resulting half-ester has a gratis carboxyl function and may then be resolvable with a chiral base, usually brucine:
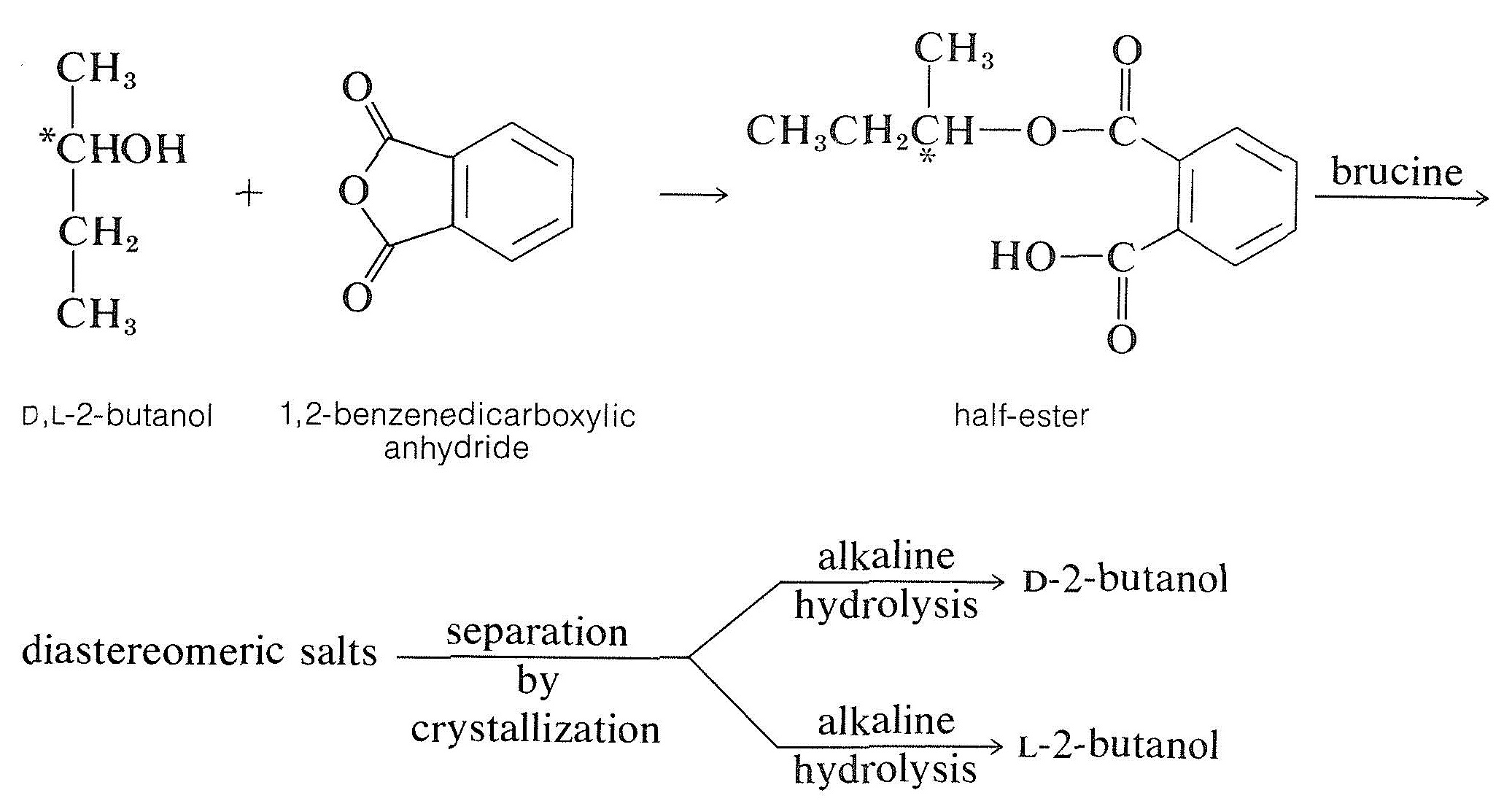
Other Methods of Resolution
One of the major goals in the field of organic chemistry is the evolution of reagents with the holding of "chiral recognition" such that they tin can bear on a clean separation of enantiomers in one operation without destroying either of the enantiomers. We take not achieved that ideal still, merely it may non be far in the future. Chromatographic methods, whereby the stationary phase is a chiral reagent that adsorbs one enantiomer more than strongly than the other, accept been used to resolve racemic compounds, but such resolutions seldom accept led to both pure enantiomers on a preparative calibration. Other methods, chosen kinetic resolutions, are fantabulous when applicable. The procedure takes reward of differences in reaction rates of enantiomers with chiral reagents. One enantiomer may react more than chop-chop, thereby leaving an excess of the other enantiomer behind. For example, racemic tartaric acid can be resolved with the assist of certain penicillin molds that consume the dextrorotatory enantiomer faster than the levorotatory enantiomer. As a result, about pure (-)-tartaric acrid can be recovered from the mixture:
(±)-tartaric acid + mold \(\rightarrow\) (-)-tartaric acid + more mold
The crystallization procedure employed past Pasteur for his classical resolution of (±)-tartaric acid (Department 5.4) has been successful but in a very few cases. This procedure depends on the formation of individual crystals of each enantiomer. Thus if the crystallization of sodium ammonium tartrate is carried out below 27", the usual racemate salt does not form; a mixture of crystals of the (+) and (-) salts forms instead. The two unlike kinds of crystals, which are related as an object to its mirror paradigm, can be separated manually with the aid of a microscope and afterwards may be converted to the tartaric acid enantiomers by potent acid. A variation on this method of resolution is the seeding of a saturated solution of a racemic mixture with crystals of one pure enantiomer in the hope of causing crystallization of just that one enantiomer, thereby leaving the other in solution. Unfortunately, very few practical resolutions have been achieved in this way.
Predicating the Chirality of the Product of a Reaction
It important to sympathize the changes in chirality which occur during the formation of product during a reaction. A chiral reaction production, has the possibility of forming multiple stereoisomers which all need to be considered. Changes in chirality, if possible, volition exist discussed with each individual reaction as this textbook moves frontward. Some possible situations which tin occur are:
- A new chiral carbon is formed during a reaction. This commonly occurs when an sp2 hybridized carbon in the reactant is converted to sp3 hybridized chiral carbon in the production. When this occurs, a racemic mixture of the new chiral carbon is formed.
- A chiral carbon is lost during a reaction. This commonly occurs when an spiii hybridized chiral carbon in the reactant is converted to either a sp2 or sp hybridized carbon in the production.
- An enantiomerically pure starting material is converted to a racemic mixture in the product. This unremarkably occurs when a spthree hybridized chiral carbon is temporarily converted to an sp2 hybrized carbon during a reaction's machinery. The chiral carbon is reformed as a racemic mixture.
- Chiral carbons remain unchanged during a reaction. If a chiral carbon is not directly involved in a reaction, it will move from a reactant to a product unchanged.
Determining if a chiral carbon is involved in a given reaction is vital for determining which of these four situations is occurring.
The following reaction involves the conversion of a carboxylic acid reacting with an alcohol to course an ester. If a pure sample of (R)-2-methylbutanoic acid is reacted with methanol to form an ester, what would be the stereochemistry of the product?
- Answer
-
First it is important to place the location of the chiral carbon and determine if it is directly involved in the reaction. In this case, the chiral carbon is not involved so the stereochemistry will be carried over into the production unchanged.
Indicate the reagents you lot could use to resolve the following compounds. Evidence the reactions involved and specify the physical method yous believe would be the best to separate the diastereomers of 1 -phenyl-2-propanamine.
- Answer
-
You could react the ane-phenyl-2-propanamine racemic mixture with a chiral acrid such as (+)-tartaric acid (R, R). The reaction will produce a mixture of diastereomeric salts (i.e. R, R, R and S, R, R). You tin carve up the diastereomers through crystallization and treat the salt with a potent base (e.g. KOH) to recover the pure enantiomeric amine.
Betoken the reagents you lot would utilise to resolve the following and discuss the reactions involved and specify the physical method you believe would be the best to separate the diastereomers of two,iii-pentadienedioic acid.
- Answer
-
You could react the 2,3-pentadienedioic acid mixture with a chiral base of operations such every bit (R)‑one‑phenylethylamine. The reaction volition produce a mixture of diastereomeric salts. Divide the diastereomers through crystallization and treat the resulting salt with stiff acid (e.g. HCl) to recover the pure enantiomeric acid.
Betoken the reagents yous would utilise to resolve the following and discuss the reactions involved and specify the physical method yous believe would be the best to separate the diastereomers of one -phenylethanol.
- Respond
-
You lot could react the 1-phenylethanol mixture with one,2-benzenedicarboxylic anhydride. The reaction will produce a mixture of diastereomeric salts. Yous could and so separate the diastereomers through crystallization and so alkaline hydrolysis handling should recover the pure enantiomeric alcohol.
Contributors and Attributions
-
Dr. Dietmar Kennepohl FCIC (Professor of Chemical science, Athabasca University)
-
Prof. Steven Farmer (Sonoma State University)
-
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. West. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted nether the post-obit conditions, "You are granted permission for individual, educational, research and not-commercial reproduction, distribution, brandish and functioning of this work in any format."
- Dr. Zachary Sharrett (Sonoma State University)
- Layne A. Morsch (Academy of Illinois Springfield)
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/05%3A_Stereochemistry_at_Tetrahedral_Centers/5.08%3A_Racemic_Mixtures_and_the_Resolution_of_Enantiomers
0 Response to "How Will You Know if You Get a Racemic Mixture"
Post a Comment